Methods
Automatic systems for measuring atmospheric CO2 mixing ratios at high absolute accuracy and precision developed by Bakwin et al.(Balwin et al., 1995, 1998; Zhao et al., 1997) are used. The systems use commercially available instrumentation and high-quality CO2 mixing ratio standard gases. This methodology was deployed on the WITN TV tower (600m tall) in North Carolina and functioned for 7 years, and has operated on the WLEF Public Television tower (447m tall) in northern Wisconsin since 1994. This method has been proven capable of approximately 0.2 ppm precision and absolute accuracy. Continuous data is acquired except during periodic automated calibration. The WLEF and WITN measurements have been extensively compared to NOAA CMDL’s flask CO2 observations (Bakwin et al., 1995, 1998; Zhao et al., 1997).
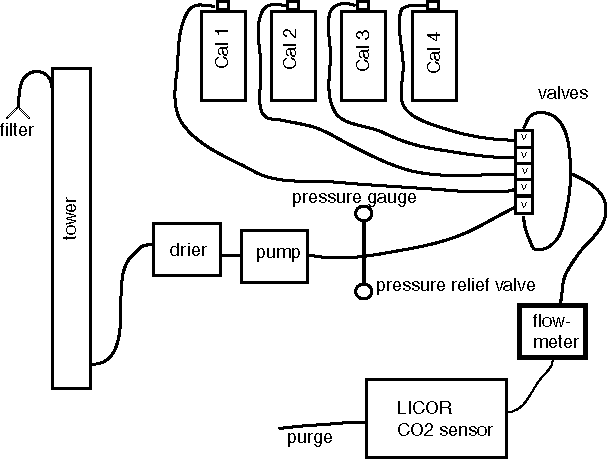
The apparatus for the measurements
For more details for parts, click <Here>

Steps for measurements
1 The sample air is dried to reduce the water vapor interference and dilution effects.
2 Analysis for CO2 mole fraction is carried out by IR absorption spectroscopy using a Li-Cor model 820 analyzer.
3 Calibration using high-quality standard gases is carried out every 2 hours, as described below.
Example of calibration procedure
Calibration is a
key step in the measurements, which is dependent upon high-quality calibration
gases (Bakwin et al,
1995,
1998 ; Zhao et al.,
1997). To
assure the stability of long-term high-precision measurements of CO2 mixing
ratio and cross-calibration of field sites, the standard gases have to be
provided via a calibration lab, such as the one set up at Penn State. This
calibration system uses four NOAA CMDL primary standard calibration tanks to
calibrate the four calibration tanks to be used at each instrumented surface
layer tower. Calibration
activities were closely coordinated with NOAA CMDL to assure high data quality.
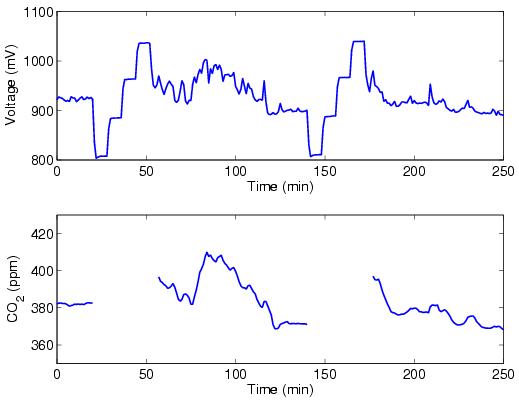
An approximately four-hour time series of raw voltages is shown in the upper
panel of the above figure. Every two hours, a calibration sequence begins in
which we sample each of four calibration tanks containing dry air with precisely
known quantities of CO2 at about 330, 360, 390, and 420 ppm. Each calibration
sequence lasts eight min for each gas, for a total of 32 min. Frequent sampling
of the calibration gases is necessary in order to account for the changing
response of the CO2 gas analyzer (primarily) with temperature and pressure.
Next a second-order polynomial describing the change in voltage with CO2
concentration is determined for each calibration cycle. For example, the
polynomial for the first calibration cycle in the above figure is [CO2] = 29.11
+ 0.35V + 0.000037V^2, where the CO2 concentration is in ppm and the voltage is
in mV. The polynomial for the second calibration cycle is [CO2] = 39.25 + 0.32V + 0.000050V^2. We use
the average of the coefficients of these two polynomials
to
determine the CO2 concentration from the voltage between the two calibration
cycles. The resulting CO2 concentration during the time in which atmospheric
air was sampled is shown in the lower panel of the above figure.
To assess the accuracy of the measurements, a set of three gases with known CO2 concentrations was tested at each site. A limited amount of flask sampling could also be adopted at a subset of flux sites if it is proven to be desirable. Another possiblity is leaving an "archive tank" at each site to test for long term stability of the measurements despite necessary calibration tank replacement (about four months of continuous operation.)